As the global supply chain continues to change, China Customs has recently imposed restrictions on the export of antimony products and antimony compounds. This has put certain pressure on the global market, especially on the supply stability of products such as antimony oxide. As China's leading sodium antimonate R&D and production company, Urban Mining Technology Co., Ltd. pays close attention to the huge potential of sodium antimonate in replacing traditional antimony trioxide (Sb₂O₃). Sodium antimonate ((Na3SbO4) has gradually replaced traditional antimony trioxide in the application of multiple industries, especially in the field of modified engineering plastics combustion additives and polyester industry catalysts (catalysts).
This article will deeply explore the principles, advantages, and industry prospects of sodium antimonate replacing antimony trioxide.
1. The basic difference between sodium antimonate and antimony trioxide
Although sodium antimonate and antimony trioxide are both antimony compounds, they have significant differences in chemical properties, physical characteristics, and application areas.
Antimony trioxide (Sb₂O₃): is one of the most common antimony compounds and is widely used in the plastics industry as a flame retardant, especially in polyvinyl chloride (PVC), polyolefins, and other engineering plastics. Its main function is to improve the flame-retardant properties of plastic materials and reduce the risk of fire. However, antimony trioxide has also gradually attracted industry attention due to its potential toxicity and impact on the environment.
Sodium antimonate (Na3SbO4): It is another important compound of antimony. It has strong antioxidant properties and does not contain toxic heavy metal components. Therefore, it is considered to be a more ideal substitute for the current increasingly stringent environmental protection requirements. Sodium antimonate is widely used in plastic modification, polyester catalysis, ceramics, glass, and other fields.
2. The principle of sodium antimonate replacing antimony trioxide
The core principle of sodium antimonate replacing antimony trioxide is mainly reflected in the following aspects:
Improve the flame retardant effect.
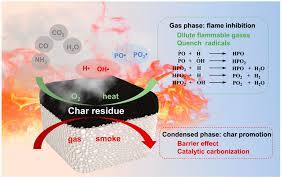
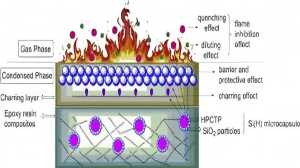
Sodium antimonate has excellent stability at high temperatures. It can react with halogen-containing polymers during plastic processing to form a solid flame-retardant film, significantly improving the flame-retardant effect of the material. As a flame-retardant additive, sodium antimonate can not only improve the flame-retardant properties of the material but also reduce the amount of smoke generated by the material in the flame, which is a clear advantage over traditional antimony trioxide.
Catalytic performance
In the polyester industry, sodium antimonate can effectively increase the polymerization reaction rate of polyester and enhance the molding efficiency of polyester fibers after being used as a catalyst (catalyst) to replace antimony trioxide, while avoiding the environmental pollution and human health risks that may be caused by traditional catalysts. Sodium antimonate as a catalyst can accurately control the reaction rate, improve product quality, and reduce waste gas emissions and the generation of by-products.
Environmental protection and safety
Unlike antimony trioxide, sodium antimonate does not contain harmful pollutants such as sulfur dioxide, and its production and use have little impact on the environment. Its safety and environmental protection make it an ideal substitute under increasingly stringent environmental regulations around the world, especially in the EU, the United States, and other regions, where environmental protection requirements have made the use of sodium antimonate more promising.
3. Advantages of Sodium Antimonate
Compared with antimony trioxide, sodium antimonate has lower toxicity and better environmental friendliness. Traditional antimony trioxide may release harmful gases during use, posing a potential threat to the health of production workers, while sodium antimonate greatly reduces this problem. It does not contain any ingredients that are harmful to the human body and meets the strict requirements of countries around the world on the use of chemicals and environmental protection.
High performance and stability
As a flame retardant additive, sodium antimonate has excellent thermal stability and antioxidant capacity. It can maintain long-term stability under high temperature and high-pressure conditions and is suitable for the processing requirements of various engineering plastics. Its catalytic performance in polyester and other polymer materials is also particularly outstanding, which can improve the reaction efficiency of polymers and thus improve the performance of the final product.
Cost-effectiveness:
The production cost of sodium antimonate is relatively stable compared to antimony trioxide, and with the continuous advancement of technology, its production process has gradually matured. For industries that require large-scale use of antimony compounds, sodium antimonate can not only provide excellent performance but also effectively reduce the environmental governance costs in the production process and improve the overall economic benefits.
Wide application:
Widely used in many industries such as plastics, catalysis, ceramics, glass, and coatings, and has great potential to replace traditional antimony trioxide. In the fields of flame retardant additives, catalysts, and other chemicals, the use of sodium antimonate is gradually becoming an industry standard.
4. Industry prospects and the role of urban mining technology
As global environmental protection requirements become increasingly stringent, especially in markets such as Europe and the United States, companies' demand for sodium antimonate continues to grow. Especially in the fields of plastics, coatings, polyesters, electronic components, etc., sodium antimonate has a broad application prospect. As a leading company in the field of sodium antimonate in China, UrbanMines Tech. Limited is committed to the research development, and production of high-purity, high-quality sodium antimonate products. The company ensures the best application effect of sodium antimonate in various industries through innovative production processes and strict quality control systems.
In the future, UrbanMines Tech. Limited will continue to promote the popularization and application of sodium antimonate products in the international market and provide global customers with safer, more environmentally friendly, and efficient solutions. At the same time, the company will increase investment in research and development to further improve the comprehensive performance of its products to meet the growing market demand for high-performance materials.
Conclusion
As a substitute for antimony trioxide, sodium antimonate is becoming the focus of attention in various industries due to its excellent environmental protection, safety, catalytic properties, and cost-effectiveness. With the increasing attention paid to environmental protection standards around the world, sodium antimonate will undoubtedly become an important part of future materials science and industrial production. UrbanMines Tech. Limited's leading position in this field will help global customers meet the challenges of antimony product supply while welcoming a greener, safer, and more efficient future.